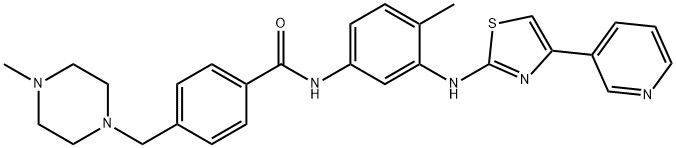
CAS Number: 790299-79-5
Standard: In-house standard
Masitinib, also known as Masitinib mesylate, is a scientific research and development of AB, a treatment for multiple myeloma, platelet-derived growth factor alpha/beta receptor tyrosine kinase inhibitors in gastrointestinal stromal tumors and prostate cancer.
Product Details

|
Molecular Formula |
C28H30N6OS |
|
Molar Mass |
498.64 |
|
Density |
1.280±0.06 g/cm3(Predicted) |
|
Melting Point |
90-95°C |
|
Solubility |
DMSO 00 mg/mL (200.54 mM);Water <1 mg/mL (<1 mM);Ethanol 4 mg/mL (8.02 mM) |
|
pKa |
13.24±0.70(Predicted) |
|
Refractive Index |
1.682 |
|
Appearance |
Power |
|
Color |
Off-White to Pale Beige |
|
Storage Condition |
Refrigerator |
Masitinib is a member of the class of Benzamides that is the Carboxamide resulting from the formal condensation of the carboxy group. It is a highly selective oral tyrosine kinase inhibitor. It has a role as a tyrosine kinase inhibitor, an antineoplastic agent and an antirheumatic drug. It is a N-alkylpiperazine, a member of 1,3-thiazoles, a member of pyridines and a member of Benzamides.
Applications
Masitinib is a potent active pharmaceutical ingredient that has been shown to be effective in the treatment of certain types of cancer and inflammatory diseases. It works by inhibiting the activity of specific tyrosine kinases, which are enzymes involved in the growth and proliferation of cells. By blocking the activity of these enzymes, Masitinib can help slow down or stop the growth of cancer cells and reduce inflammation.
Masitinib is used as the main active ingredient in medications that are approved for the treatment of advanced gastrointestinal stromal tumors and systemic mastocytosis in Europe. It is still undergoing clinical trials for other potential uses, including the treatment of amyotrophic lateral sclerosis (ALS), Alzheimer's disease, and multiple sclerosis.
As an active pharmaceutical ingredient, Masitinib must be carefully formulated and manufactured to ensure its purity, potency, and stability. This involves strict quality control processes and adherence to good manufacturing practices. Other ingredients, such as fillers and binders, may also be added to create the final medication product. Overall, Masitinib represents a promising therapeutic option for several diseases and conditions, and ongoing research will likely shed more light on its potential benefits and limitations.
Customer Questions
Q: Where is your factory located?
A: It is located in Torch Hi-tech Industrial Development Zone, Xiamen, Fujian, China.
Q: When was your factory established?
A: It was established in 2013.
Q: What is your main market?
A: Origin often ships to Europe, South America, the Middle East, and Asia, and has received universal praise from both local and international consumers.
Hot Tags: masitinib for veterinary cas 790299-79-5, China masitinib for veterinary cas 790299-79-5 manufacturers, suppliers, factory, Denaverine Hydrochloride, 790299-79-5, Toceranib, 69770-45-2, Trilostane, Maropitant Citrate